The New Year has started with numerous new, revised, and revisited regulations and requirements – some starting this year and others that began in late-2017. Below is a brief reminder of some of the important issues that long-term care providers should have on their radar.
New Medicare Identification Numbers
Starting in April 2018, new cards will be sent to beneficiaries, eliminating the social security numbers from their Health Insurance Claim Numbers (HICN). CMS has allotted 12 months for the millions of Medicare beneficiaries to receive their new Medicare Beneficiary Identifier (MBI) card.
The transition for using the new MBIs for the transferring of information will begin April 1, 2018. This means that software programs must be updated and tested to ensure that the new numbers are accepted. Note that persons just becoming Medicare eligible and receiving a Medicare number for the first time will only receive the MBI starting April 1, 2018.
There is a difference between the new MBI for Medicare and those receiving benefits through Railroad Retirement Board. It is essential to be able to identify these two beneficiaries. Those receiving benefits from the Railroad Retirement Board will had the RRB logo in the left upper corner of the card, and RAILROAD RETIRMENT BOARD indicated below the eligibility dates. Your system must be able to discern between the two.
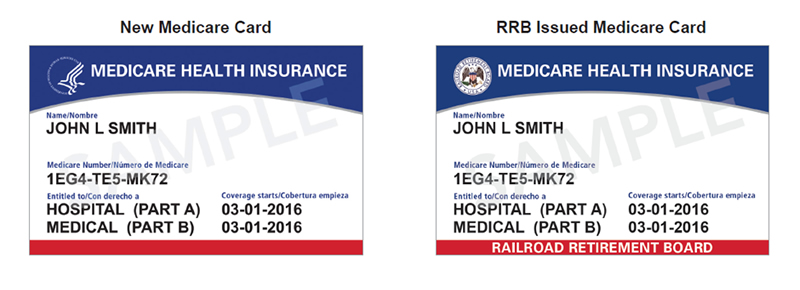
All MBIs are randomly selected and will include a combination of 11 letters and numbers – spouses and dependents may have a totally different number that the original beneficiary.
More information can be located on the CMS website.
SNF QRP Full Confidential Feedback Reports
The most current data is now available for review and correction. This report contains both assessment and claims quality measures data that will be used to determine the providers ranking for public reporting in October 2018. It is important that the information be reviewed and any error noted be sent back to CMS with documentation to support what is felt to be wrong information. As a reminder, the confidential reports are available through the CASPER system and the current reports use data from 10/1/16-9/30/17.
More information can be located on the CMS website.
Section GG Training is Available
If your staff is still confused about coding Section GG, CMS has posted Web-based training modules, which are available on demand, and delve into specific coding tips, definitions, and timing issues that are important for the provider’s staff to know. In addition, a transcript is available that can readily be used to educate other interdisciplinary team members.
More information about the program is located on the CMS website.
Updated G Code Chart
CMS and the Medicare Learning Network have updated and posted a Quick Reference Chart for Therapy Functional Reporting. This educational tool may be found on the CMS website.
As a reminder, the G codes apply to Medicare-Fee-For Service only.
RCS-1
Ready or not, we must be prepared for the change in the Medicare reimbursement methodology—a change must be made by October 2018-that is this year. Although a proposed rule has yet to be published, those in the know are preparing for the Resident Classification System (RSC), in some version, to take place. This system bases Medicare payments on value rather that volume and, if it in fact goes into effect, it will require day-to-day operations in our facilities to change.
In anticipation of this new method of reimbursement, facilities should ensure that their MDS coordinators, or those who complete the diagnosis portion of the MDS and UB04, hone their skills on proper and accurate ICD-10 coding. Much of the reimbursement is based on diagnosis. If Section I8000 has been ignored, is inaccurate, or incomplete, many dollars will be lost under the new system.
Secondly, MDS accuracy and documentation to support MDS coding is essential. Now is the time to do some mock chart audits to see where your facility stands in the documentation arena. A few questions that might help to answer where your facility falls are:
- Is there adequate documentation to support depression and behavior codes in Section D if the PHQ interview is not completed? What about for Section E?
- Does therapy documentation and nursing notes contradict each other? If so, is there justification?
- Does the dietary assessment and CNA documentation contradict each other? If so, why?
- How are decision making skills, long and short term memory, and recognition documented in the clinical record, and does it support MDS coding if a BIMS interview is not completed?
- Are interviews attempted when communication is not impaired?
New Long-Term Care Survey Process
November 28, 2017 marked the start of the new survey process which includes all the Phase 2 requirements of the Requirements of Participation. Although there is an 18-month moratorium on certain enforcement remedies (civil monetary penalties, discretionary denials of payment for new admissions, and discretionary termination where the remedy is based on a specific phase 2 F Tag; see list below), the facility may still be cited.
This will impact the history of surveys for the facility, and although it will not impact the 5-Star rating at this time, the results will be part of the survey history that is used when determining the Health Inspection portion of the 5-Star program.
The tags included in the moratorium are:
- Baseline Care Plans (F655).
- Behavioral Health Services (F740).
- Sufficient/Competent Direct Care/Access Staff-Behavioral Health (F741).
- Psychotropic Medications (F758) related to PRN limitations.
- Facility Assessment (F838).
- Antibiotic Stewardship (F881).
- QAPI Program and Plan (F865) related to the development of the QAPI plan.
- Smoking Policies (F926).
Be aware that other enforcement remedies may be imposed if warranted and under certain conditions. These include Directed Plan of Correction and/or a Directed In-Service Training. The regional office may use these if Phase 1 and Phase 2 Tags are identified. Therefore, it is not prudent to leave everything in limbo for the Phase 2 requirements for the next 18 months.
More information of the new process is located here on the CMS website; for the moratorium here on the CMS website.
Please contact a Marcum LLP healthcare associate with any questions.